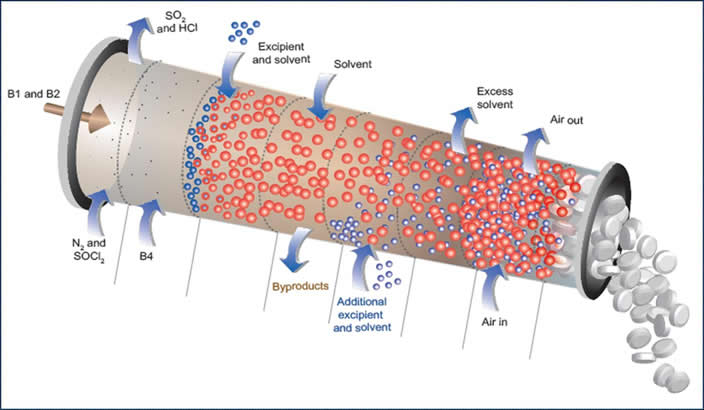
Continuous Pharmaceutical Manufacturing
Currently, pharmaceutical products are manufactured primarily using batch processes, as has been the industry standard from the beginning. There is, however, tremendous potential to transform the entire industry by producing these products via continuous manufacturing. Moving to continuous has the potential for huge increases in efficiency, flexibility, and quality. As part of the Novartis-MIT Center for Continuous Manufacturing, a major effort in the Trout Group, together with the Hatton Group, focuses on the development of new technologies to effect continuous pharmaceutical manufacturing. These include continuous crystallization processes together with a variety of novel separations and final finishing processes that have the potential to transform pharmaceutical manufacturing.
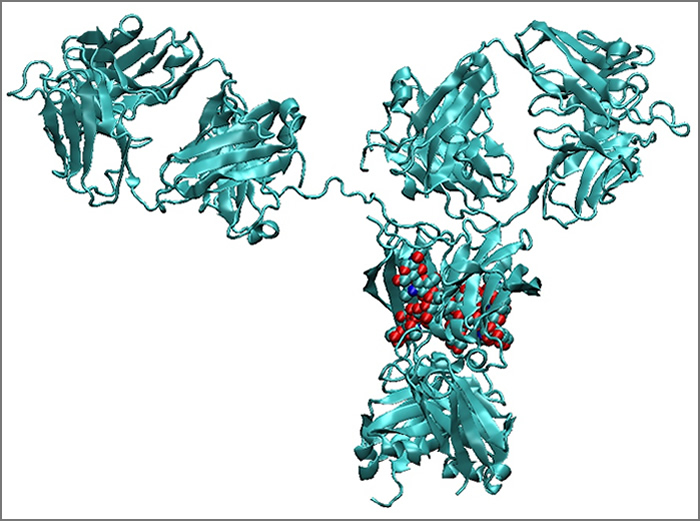
Stabilization and Formulation of Biopharmaceuticals
Biopharmaceutical, including antibodies constitute the most rapidly growing class of human therapeutics for the treatment of numerous indications, including cancer, chronic inflammatory diseases and infectious diseases. One of the major problems encountered in biopharmaceutical therapies is the inherent instability of biopharmaceuticals to degradation processes, such as aggregation, oxidation, hydrolysis, and deamidation. Our group has a major effort in developing a mechanistic and quantitative understanding of these processes, leading to new approaches to stabilizing biopharmaceuticals.
Part of our work involves developing a mechanistic understanding how therapeutic antibodies aggregate during long-term storage. We employ molecular simulation tools in collaboration with experimental techniques to elucidate aggregation mechanisms and determine molecular engineering strategies for antibody stabilization.
Nucleation and Crystallization
Solution crystallization is a commonly used technique in the pharmaceutical industry to separate and purify API’s (Active Pharmaceutical Ingredients). Understanding of crystallization together with nucleation is primarily based on heuristics and rough macroscopic models. We aim to better control these processes via the development of molecular-based understanding. Such understanding has the potential to make rational the solvent selection process and design of crystallization processes and therein streamline pharmaceutical development.
The Molecular Engineering Laboratory at MIT was founded to develop cutting edge molecular computational methods and apply these methods to address important industrial problems. Thus, a major aspect of the work in the Trout Group involves new molecular computational approaches based on statistical mechanics and quantum mechanics. We are pursuing a number of new approaches to find reaction coordinates in complex systems, including the ones described above.